3cus 8hno3 3cu No3 2 2no 3s 4h2o, 4.20f | What atoms are oxidized and reduced: 3Cu(s) + 8HNO3(aq) ? 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(l), 23.32 MB, 16:59, 1,743, The Glaser Tutoring Company, 2021-11-12T16:00:21.000000Z, 15, PPT - ? 10 ? ?????? precipitation-dissolution equilibrium PowerPoint, www.slideserve.com, 1024 x 768, jpeg, , 5, 3cus-8hno3-3cu-no3-2-2no-3s-4h2o, KAMPION
Temas 4.20f | What atoms are oxidized and reduced: 3Cu(s) + 8HNO3(aq) ? 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(l) popular
Veamos ? 8HNO3 + 3Cu = 3Cu(NO3)2 + 2NO + 4H2O Calcula el volumen del Oxido Nitroso
Explicación de 3cus 8hno3 3cu No3 2 2no 3s 4h2o en su totalidad
Identify the atoms that are oxidized and reduced, the change in oxidation state for each, and the oxidizing and reducing agents in each of the following equations:
3Cu(s) + 8HNO3(aq) ? 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(l)
OpenStax™ is a registered trademark, which was not involved in the production of, and does not endorse, this product.
If you don't have the OpenStax™ "Chemistry: Atoms First" textbook, here is a link in which you can download it for FREE!
d3bxy9euw4e147.cloudfront.net/oscms-prodcms/media/documents/ChemistryAtomsFirst2e-OP_T2wT7wj.pdf
SUBSCRIBE if you'd like to see more solutions for your textbook!
youtube.com/channel/UC2C34WdYMOm47PkWovvzLpw?sub_confirmation=1
Want us as your private tutor? Get started with your FREE initial assessment!
glasertutoring.com/contact/
#OxidationStates #RedoxReactions #OpenStaxChemistry
Imágenes PPT - ? 10 ? ?????? precipitation-dissolution equilibrium PowerPoint volviéndose viral

PPT - CuS+HNO 3 ?Cu(NO 3 ) 2 + S + NO+H 2 O PowerPoint Presentation
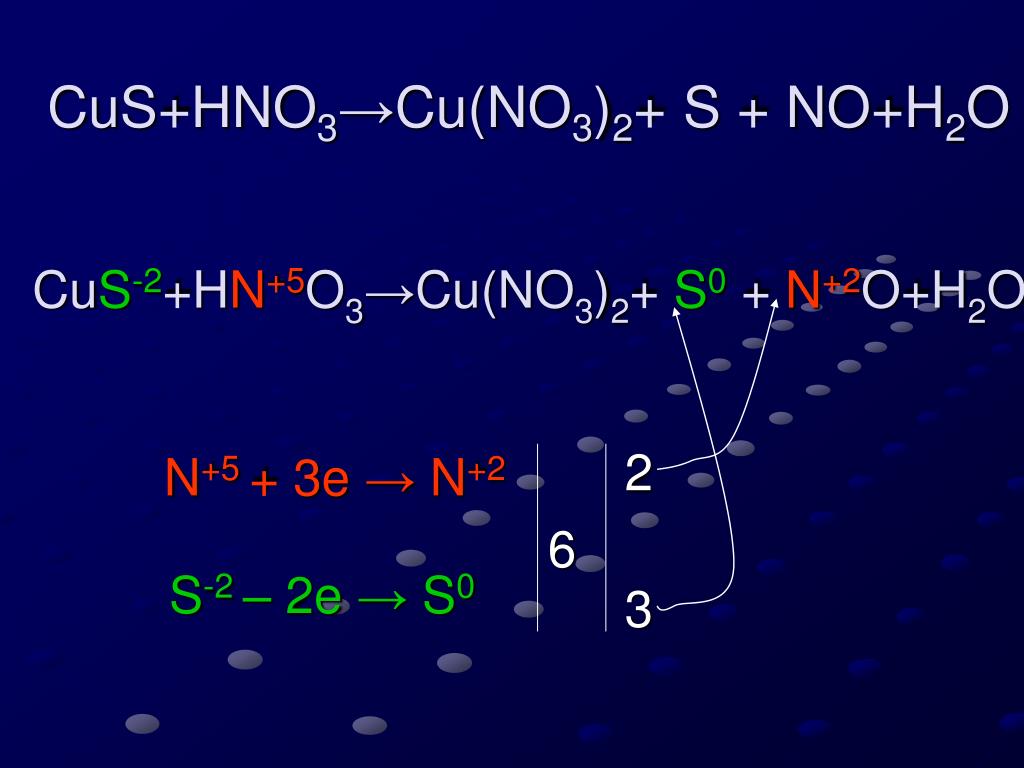
Artículos PPT - CuS+HNO 3 ?Cu(NO 3 ) 2 + S + NO+H 2 O PowerPoint Presentation
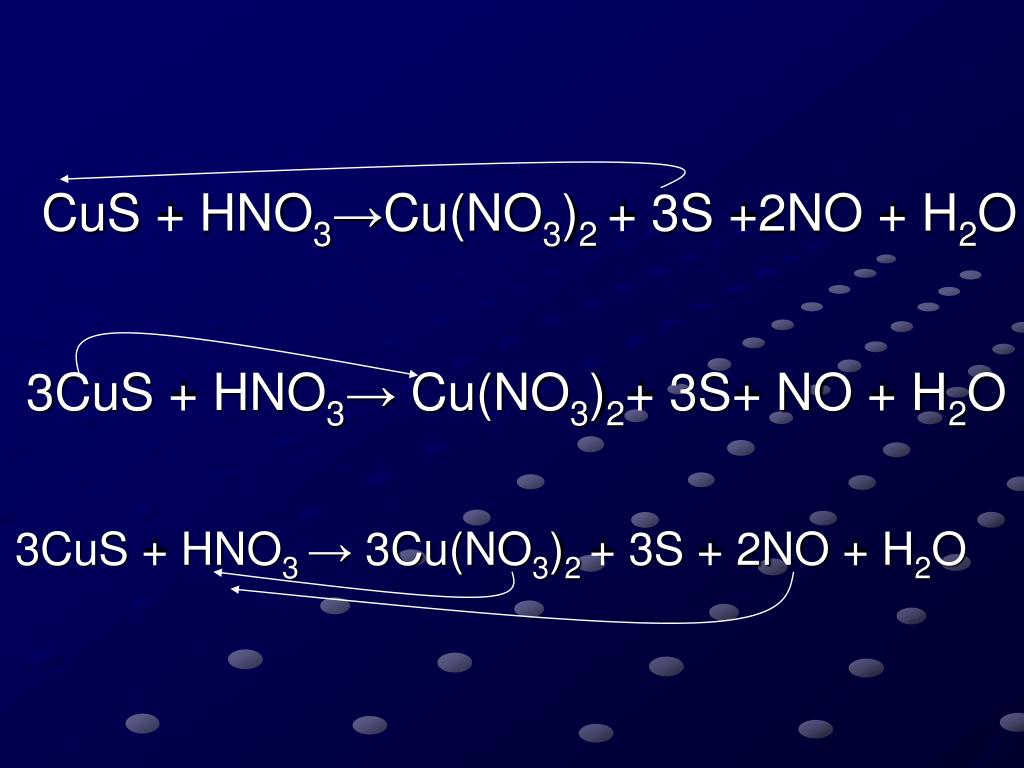
Reseñas Clase 5 quim. inorganica cualitativa volviéndose viral

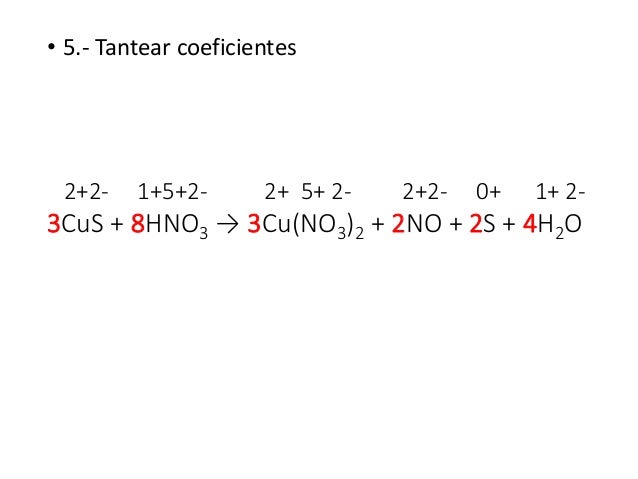
Ver Balanceo de ecuaciones por método de redox Javier



0 Comments